About the Design of the Lasix ONYU Infusor
The Infusor was designed specifically for the administration of furosemide to patients with heart failure. We considered the needs of the patient, the provider, the payor and the environment.
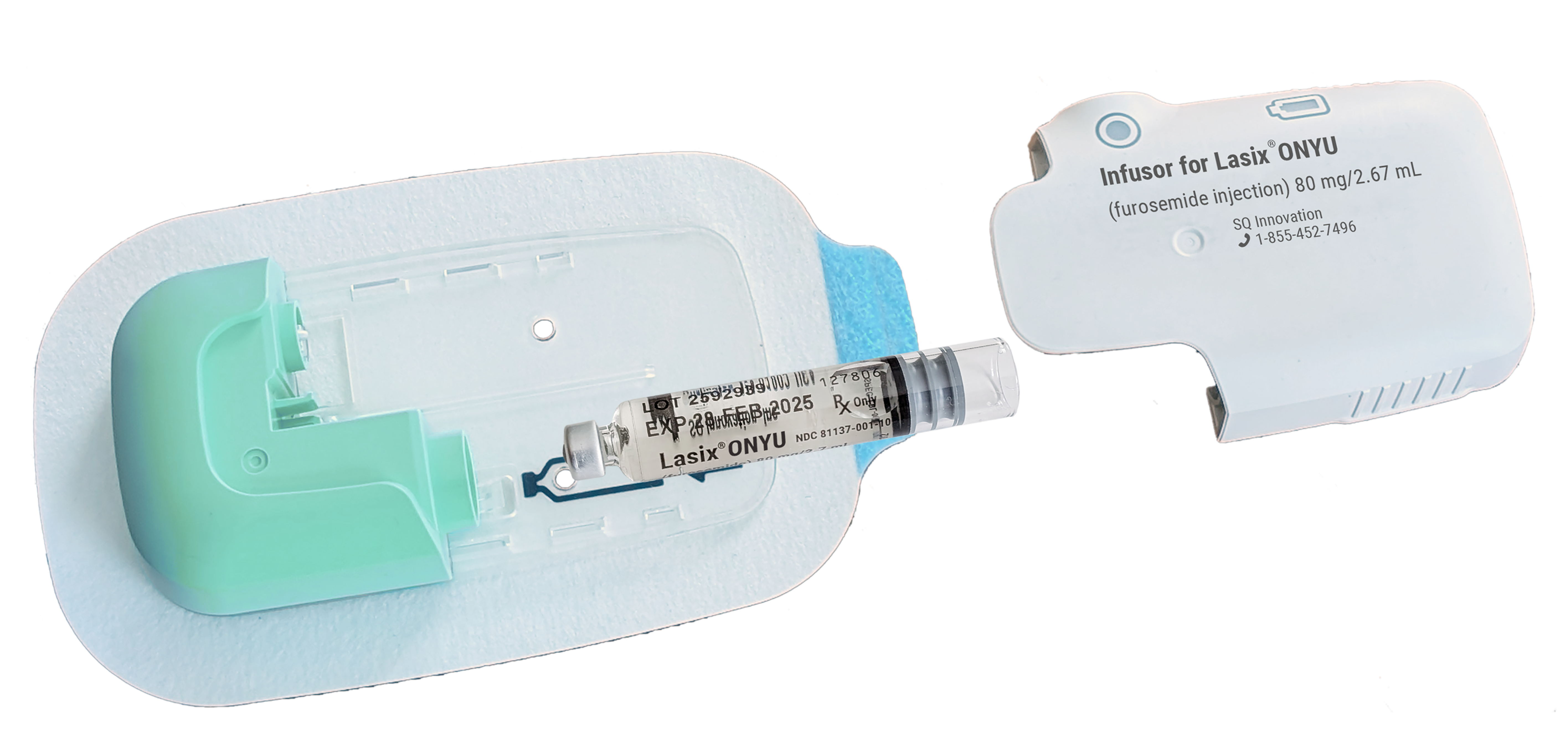
The Infusor has three components:
- Reusable Unit: A personal device that can be used up to 48 times. It contains a small electromotor, battery and electronics. It is charged before each each use, which takes less that 10 minutes.
- Disposable Unit: A sterile single use plastic component that includes everything in contact with the drug or body, including a very thin needle for the infusion. The needle comes out when the infusion starts and retracts at completion.
Drug Cartridge: A prefilled glass cartridge containing 80mg furosemide in 2.67mL (30mg/mL).
Read Important Safety Information and the step-by-step instructions in the Instructions for Use before using the Infusor.

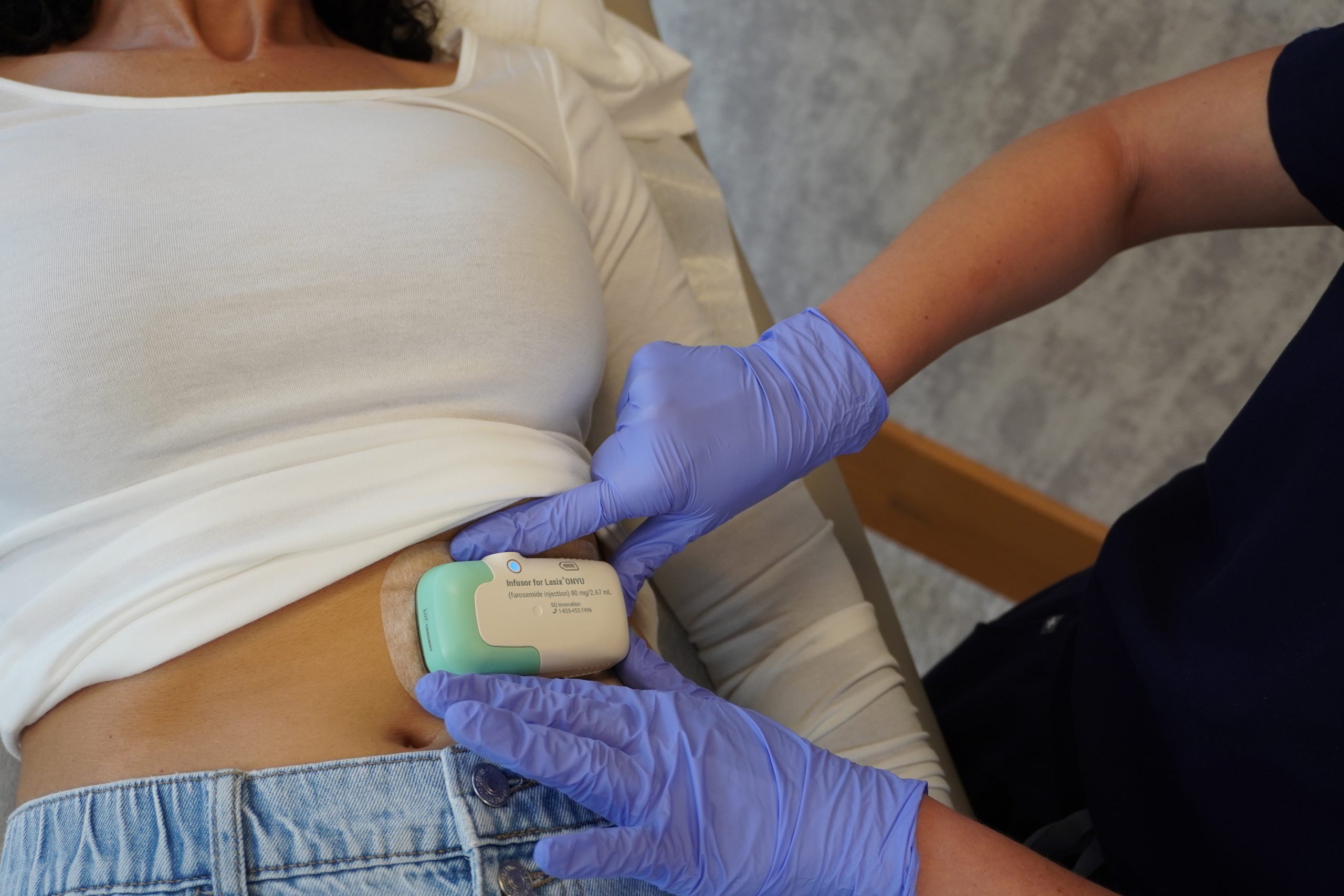
Designed for the Patient
The patient is our most important customer. First, we wanted the Infusor to be small and light for comfort during use, and so we could use a more gentle medical tape. This may sound trivial, but the abdominal skin of the elderly is fragile and removing more aggressive tape may hurt and cause bruising or skin damage. We are using a 3M medical tape that has been used since 1980 and is known to be one of the most gentle medical tapes. Unfortunately, some patients may still have a reaction, which in most cases is slight redness that goes away quickly (See Important Safety Information and Prescribing Information).
Designed for the Provider
Treating worsening heart failure and decongestion have clinical workflows. We wanted our new treatment to fit how clinicians want to take care of patients with heart failure. One of the most common workflows is that the patient with congestion due to fluid overload will be seen in the outpatient clinic or Emergency Department. Some patients are too sick or going home is not an option. These patients need to be admitted for treatment. However, there are also patients who have bothersome symptoms due to fluid overload and who just need parenteral diuretic treatment. In other words, there is no other clinical reason for admission and the patient is a good candidate to be treated at home. In many cases, clinicians want to instruct the patient there and then. The best ways of doing this is by preparing the first treatment together. Our design allows training of the patient, preparing and placing the device and then having the patient travel home. Upon arriving home, the patient can press the button and start treatment. Our Infusor allows for 7 hours between placement and starting therapy — more than enough time for almost every patient to reach the comfort of their home.

Designed for the Payor
Money for healthcare is tight. Widespread adoption and easy availability requires that payors also benefit. At home treatment with Lasix ONYU should be one of the most affordable options available when the patient is a good candidate. Electronics are expensive and the materials they use are scarce. Patch pumps like the Infusor contain a motor, controllers (chips), LEDs, and a battery. Our Infusor can be used up to 48 times, after which it can be recycled. The DU is manufactured on a high-capacity robotic line. All combined, this allows Lasix ONYU to be affordable to the benefit of patient and payor.
Designed for the Planet
Medical waste is incinerated and ends up in landfills. This includes single-use medical products with electronics. This is also an important reason why we selected the two-component design. Our two-component design assures that the electronic components and the battery are not part of the medical waste. First, the electronics can be used for 48 treatments and second, the electronics can be recycled. We also wanted to avoid chemical sterilization. Medical devices with electronic components are commonly sterilized with chemicals, most commonly ethylene oxide, a known carcinogen. Because of these concerns, the FDA, the US Department of Labor and the Environmental Protection Agency all promote alternatives to ethylene-oxide. Because the sterile single use component (DU) does not contain electronics, we can safely sterilize with radiation and avoid the chemicals.


